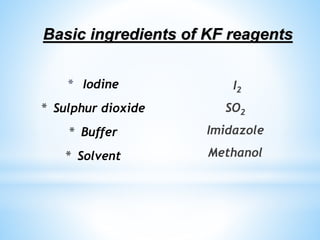
This document discusses Karl Fischer titration, which is a method for determining the water content in a sample. It involves titrating the sample with a Karl Fischer reagent, which contains iodine, sulfur dioxide, and an alcohol solvent. There are two main types of Karl Fischer titration: coulometric titration, where iodine is generated in the titration cell, and volumetric titration, where a solution with a known iodine concentration is used as the titrant. The endpoint is detected electrochemically. Karl Fischer titration is used in various industries to measure water content in products like oils, plastics, gases, cosmetics, pharmaceuticals, and food. A limitation is that the