Volumetric analysis-Solution stoichiometry
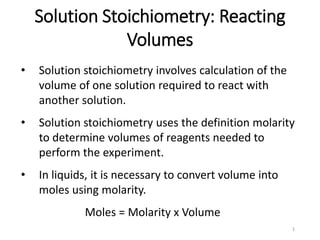
- 1. 1 Solution Stoichiometry: Reacting Volumes • Solution stoichiometry involves calculation of the volume of one solution required to react with another solution. • Solution stoichiometry uses the definition molarity to determine volumes of reagents needed to perform the experiment. • In liquids, it is necessary to convert volume into moles using molarity. Moles = Molarity x Volume
- 2. 2 Example: Reacting Volumes • A 0.0823 M solution of sodium chloride (NaCl) is added to 21.40 mL of 0.962 M silver nitrate (AgNO3) solution forming a precipitate of silver chloride. Calculate the minimum volume of the sodium chloride solution required for complete reaction. Solution; • First come up with the balanced chemical equation and interpret it using the mole method.
- 3. 3 Example: Reacting Volumes • Mole interpretation: 1 mol NaCl reacts with 1 mol AgNO3 to produce 1 mol AgCl • Calculate the number of moles of the reactant with known molarity and volume using the relationship: Moles = Molarity x Volume • The reactant with known volume and molarity is silver nitrate (21.40 mL of 0.962 M of AgNO3).
- 4. 4 Example: Reacting Volumes • Calculate the number of moles of the second reactant using the stoichiometric coefficients of the balanced equation. – The balanced chemical equation tells us that 1 mol NaCl reacts with 1 mol AgNO3. – Therefore the number of moles of NaCl required to react with 0.0206 mol AgNO3 will be 0.0206 mol. • Calculate the volume of the second reactant using the equation:
- 5. 5 Practice: Reacting Volumes 1. Sodium hydroxide (NaOH) reacts with sulphuric acid (H2SO4) according to the following equation: 2NaOH (aq) + H2SO4 (aq) → 2H2O (l) + Na2SO4 (aq) Find the volume of 0.112 M NaOH solution required to completely react with 10.0 mL of H2SO4 solution. 2. Calcium hydroxide is sometimes used in water treatment plants to clarify water for residential use. Calculate the volume of 0.0250 mol/L calcium hydroxide solution that can be completely reacted with 25.0 mL of 0.125 mol/L aluminum sulfate solution. Al2(SO4) (aq) + 3 Ca(OH)2 (aq) → 2 Al(OH)3 (s) + 3 CaSO4 (s)
- 6. 6 Volumetric Analysis • Volumetric analysis is the analytical technique for determining the amount of a certain substance in the solution by measuring the volume of a reacting solution. • In the volumetric analysis, the concentration of a solution of unknown concentration is worked out from the volume of a solution of a known concentration. • Technique involves working out the amount of one reactant from the volume of the solution and its conc’. Then the amount worked out is converted to the amount of the other reactant using the stoichiometric relationship from the balanced chemical equation.
- 7. 7 Volumetric Analysis: Example • 25.00 mL of phosphoric acid (H3PO4) solution required 27.55 mL of a 0.155 M potassium hydroxide (KOH) solution for neutralization. Calculate the molarity of the phosphoric acid The equation for the reaction is: H3PO4 (aq) + 3 KOH (aq) → K3PO4 (aq) + 3 H2O (l) Solution; • From equation: 1 mol H3PO4 reacts completely with 3 mol KOH
- 8. 8 Volumetric Analysis: Example • Calculate the number of moles of the reactant with known molarity and volume (i.e. KOH): • Using the stoichiometric coefficients of the balanced equation, calculate the number of moles of H3PO4 required to completely react with 4.27 x 10-3 mol KOH. Thus;
- 9. 9 Volumetric Analysis: Example • Therefore 1.42 x 10-3 mol H3PO4 were present in 25.00 mL of H3PO4 • Calculate the molarity of the H3PO4 using the equation:
- 10. 10 Volumetric Analysis: Practice • Ammonium sulfate is manufactured by reacting sulfuric acid with ammonia according to the reaction equation: H2SO4 (aq) + 2 NH3 (aq) → (NH4)2SO4 (aq) Find the concentration of sulfuric acid required to react with 24.4 mL of a 2.20 mol /L ammonia solution if 50.0 mL of sulfuric acid is used.
- 11. • Titration: the process of analyzing composition by measuring the volume of one solution needed to completely react with another solution. This is a special case of a Limiting Reagent! Volumetric Analysis (Titrimetry)
- 12. • In a titration, a solution of accurately known conc’ is added gradually to another solution of unknown concentration until the chemical reaction between the two solutions is complete. • Analyte: the solution of unknown concentration but known volume. • Titrant: the solution of known concentration Analyte + Titrant → Products ❖ Add titrant until all of the analyte has reacted ( then need to detect excess of titrant). Titration
- 13. 13 • Equivalence point – the point where the amount of titrant added is enough to completely neutralize analyte solution. • Indicator – substance that changes colour at (or near) the equivalence point (i.e. when excess titrant has been added). • End point: the point where the indicator changes its colour. Slowly add base to unknown acid UNTIL the indicator changes color E.g. for reaction of an acid with a base. Titration
- 14. Titration Setup
- 15. 15 Titration Calculations 1. Find the number of moles of titrant added to reach the endpoint. 2. Determine the moles of analyte that must have been present (use stoichiometric coefficients). 3. Determine the concentration of analyte that must have been present (use the volume of analyte).
- 16. 16 Titrations can be used in the analysis of • Acid-base reactions • Redox reactions H2SO4 + 2NaOH 2H2O + Na2SO4 5Fe2+ + MnO4 - + 8H+ Mn2+ + 5Fe3+ + 4H2O • Precipitation reactions Ag+ (aq) + Cl- (aq) AgCl (s) 2Ag+ (aq) + CrO4 2- (aq) Ag2CrO4 (s)
- 17. An acid-base titration (titrating an acid with base). Start of titration Excess of acid Point of neutralization Slight excess of base At equivalence point : moles H+ = moles OH-
- 18. 18 What volume of a 1.420 M NaOH solution is required to titrate 25.00 mL of a 4.50 M H2SO4 solution? WRITE THE CHEMICAL EQUATION! volume acid moles acid moles base volume base H2SO4 + 2NaOH 2H2O + Na2SO4 M acid rxn coef. M base n(H2SO4) = C(M) x V n(H2SO4) = 0.025 L x 4.5 mol/L = 0.1125 mol From stoichiometry 2 mol NaOH = 1 mol H2SO4 n(NaOH) = 2 x n(H2SO4) = 2 x 0.1125 = 0.225 mol C (M) n V = 1.420 M 0.225 mol V = = 158 mL NaOH
- 20. 20 16.42 mL of 0.1327 M KMnO4 solution is needed to oxidize 25.00 mL of an acidic FeSO4 solution. What is the molarity of the iron solution? WRITE THE CHEMICAL EQUATION! volume red moles red Moles oxid Volume oxid M acid rxn coef. M base n(MnO4 -) = C(M) x V n(MnO4 -) = 0.01642 L x 0.1327M = 0.0022 mol From stoichiometry 5 mols Fe2+ = 1 mol MnO4 - n(Fe2+) = 5 x n(MnO4 -) = 5 x 0.0022 = 0.011 mol V n C (M) = 0.025 L 0.011mol = = 0.4358 M FeSO4 5Fe2+ + MnO4 - + 8H+ Mn2+ + 5Fe3+ + 4H2O 16.42 mL = 0.01642 L 25.00 mL = 0.02500 L
- 21. 1.065 g of H2C2O4 (oxalic acid) requires 35.62 mL of NaOH for titration to an equivalence point. What is the concentration of the NaOH? Part A: Standardize a solution of NaOH — i.e., accurately determine its concentration. Step 1: Calculate amount of H2C2O4 90.04 g/mol 1.065 g n = M m n = = 0.0118 mol Step 2: Calculate amount of NaOH required H2C2O4(aq) + 2 NaOH(aq) ---> Na2C2O4(aq) + 2 H2O(liq) acid base From stoichiometry 1 mol H2C2O4 = 2 mols NaOH n(NaOH) = 2 x n(H2C2O4) = 2 x 0.0118 = 0.0236 mol NaOH Step 3: Calculate concentration of NaOH V n C (M) = 0.03562 L 0.0236 mol = = 0.663 M NaOH
- 22. Apples contain malic acid, C4H6O5. 76.80 g of apple requires 34.56 mL of 0.663 M NaOH for titration. What is weight % of malic acid? Part B: Use standardized NaOH to determine an amount of acid Step 1: Calculate amount of NaOH used n = C. V = 0.0229 mol Step 2: Calculate amount of malic acid titrated C4H6O5(aq) + 2 NaOH(aq) ---> Na2C4H4O5(aq) + 2 H2O(l) From stoichiometry 1 mol C4H6O5 = 2 mols NaOH n(C4H6O5) = 1/2 x n(NaOH) = 1/2 x 0.0229 = 0.0115 mol Step 3: Calculate the mass of malic acid titrated = 1.54 g n = 0.663 M x 0.03456 L m = n.M = 0.0115 mol x 134 g/mol
- 23. Apples contain malic acid, C4H6O5. 76.80 g of apple requires 34.56 mL of 0.663 M NaOH for titration. What is weight % of malic acid? Step 4: Calculate weight percentage of malic acid = 2.01 % m (apple) m (malic acid) % malic acid = x 100% 76.80 g 1.54 g % malic acid = x 100%
- 24. aA + bB products Remember that both A and a are on top and both B and b are on the bottom A general expression Consider a general reaction: Therefore From stoichiometry: Amount (mol) A Amount (mol) B a b = But we also know that: Amount of A in mols, nA = CAVA Amount of B in mols, nB = CBVB [A] VA [B] VB a b = CA VA CB VB a b =